Alzheimer’s disease is both debilitating and fatal. Its associated memory loss is more than a sign of normal aging, and Alzheimer’s is a leading cause of death in the United States. While the pace of Alzheimer’s treatment research is impressive, it is mirrored by a monumental projected increase in prevalence over the next 30 years.
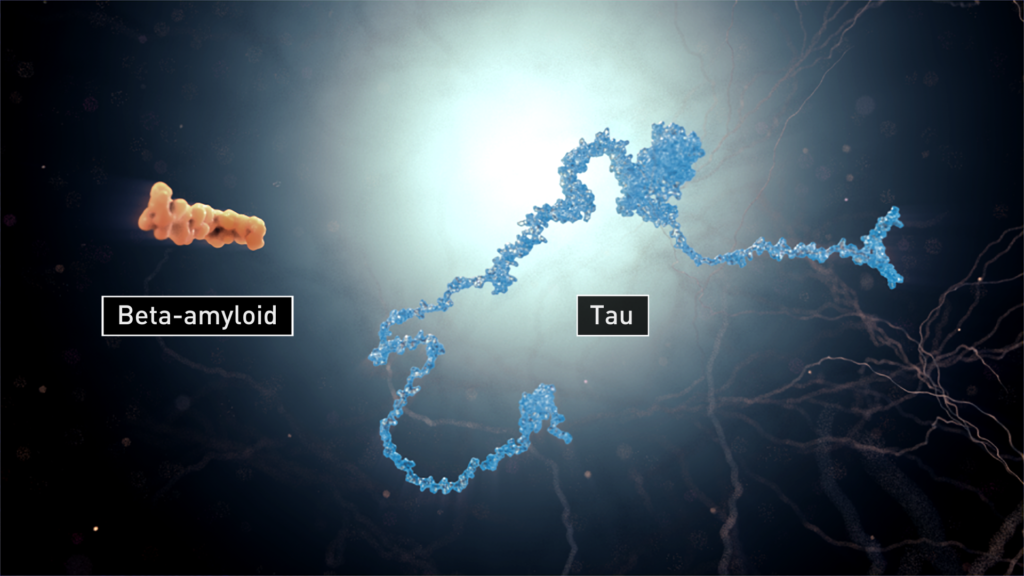
A key aspect of developing effective treatments for Alzheimer’s, and for any disease, is to understand how the disease progresses. One of the causes of Alzheimer’s is believed to correlated with malfunctioning tau proteins in our nerve cells, or neurons. Tau proteins, when under a normal function, stabilize neuron’s microtubules serving as information carriers. When tau proteins malfunction, the nerve cells reject the accumulated debris of tau and spit it out. Another small section of protein, beta-amyloid peptides, are involved in neuronal structure as well as in Alzheimer’s disease. Scientists believe the nasty feedback loop in which beta-amyloid’s failure would trigger tau protein malfunction and that tau tangles, in turn, enhance beta-amyloid toxicity. These extracellular proteins stick together in bundles, forming strands of tangles and clusters of plaques around the dying neurons.
Research out of the University of Cambridge, published in Brain this month, combined functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) scans to visualize the location and extent of tau proteins in the brains of 17 Alzheimer’s patients and 12 controls. They also included 17 patients with progressive supranucelar palsy (PSP), a brain disorder sometimes mistaken for Parkinson’s disease.
The Cambridge researchers wanted to test the theory of “transneuronal spread” – that tau tangles from one neuron pass to other neurons, rather than simultaneously occur at multiple locations. The imaging from their research study showed that abnormal tau proteins seemed to accumulate along heavily connected regions of the brain, where neurons are densely connected to one another. This theory supports the progressive nature of Alzheimer’s with an increasing loss of functional connectivity, and the researchers are planning additional studies to follow these patients over time and document the spread of these proteins. Animal research has previously shown tau propagation via synaptic connectivity in rodent models.
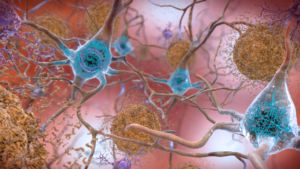
Interestingly, the Cambridge researchers found evidence of a different tau propagation in progressive supranucelar palsy (PSP) patients. The spread of tau tangles seemed to follow increased metabolic demand, not functional neuronal connectivity. This different mechanism may explain the different symptoms seen in Alzheimer’s, often presenting as memory loss, and PSP, often presenting as weakness.
Want to learn more about Alzheimer’s disease? Take an interactive tour of the brain and learn how the disease occurs and progresses at the physiological level.
Peer edited by Chiung-Wei Huang and Kate Newns.
Follow us on social media and never miss an article: