In July of 2019, just months before the world came to a halt in the name of a global pandemic, I sat on a bench in front of the Kulem Mountain Village Health Center in rural Cambodia. It was the only health center within miles of the rural town and its six neighboring communities– all seven of which relied on a rotating staff of two midwives and a nurse nearing retirement. The facility had a small check-in desk, a couple of delivery beds half the size of the patient beds that UNC’s medical students practice with, and a small refrigerator meant for the occasional delivery of vaccines. Even with a small medical team and limited resources, the health center, like many others found across rural Cambodia, has made strides in efforts against the nation’s most prevalent communicable diseases: HIV, tuberculosis, and malaria. Although their resilience and dedication to support the health of their communities is incredibly admirable, the social, economic, and health disparities are unjust when recognizing the necessity of health equity. This includes equitable access to technologies like the COVID-19 vaccine. I sit here having received my jabs of Pfizer, wondering how long it will be until the fridge on that mountain top fills with vaccines against COVID-19 for the 6,000 Cambodians on Mount Kulem. In a position of privilege, it is easy to lose sight of the fact that this reality is not a world away– that this pandemic isn’t over until we achieve health equity for all.

Over the past year, the world witnessed what seems like the miraculous development of vaccines against COVID-19. By now, we have heard the origin story of the mRNA vaccine technology and the years of research that went into some of the leading COVID-19 vaccines in the United States. To date, approximately 57% of the US population is fully vaccinated, and approximately 45% of the global population has received a full course of COVID-19 vaccines. However, the distribution of the vaccines has been far from equitable with 52 of the least wealthy countries having received just over 4% of the vaccinations.

Wealthier countries including Australia, Canada, and the United States negotiated deals with vaccine manufacturers early-on, to ensure that they would have a sufficient supply for their citizens. High-income countries are currently administering COVID-19 vaccines at a rate 20x faster than their lower-income counterparts. This leaves lower-income countries especially vulnerable to the virus as they are unable to immunize the vast majority of their populations. As we have witnessed time and time again over the past two years, borders mean nothing when it comes to the transmission of this virus and its variants. The Director-General of the World Health Organization, Dr. Tedros Adhanom Ghebreyesus, has stated that the inability to ensure that the most vulnerable nations have equitable access to the vaccines is “epidemiologically self-defeating.”
International collaboration is necessary to safeguard the health of people across the globe and bring an end to this pandemic. Several high-income countries have stepped up to the plate by pledging doses to countries in need. As of June 2021, the United States has pledged over 587 million doses; the UK has pledged 100 million doses; France, Germany, and Japan have pledged 30 million each; and China has already donated 30 million doses to over 59 countries. A projected 11 billion doses are needed to fully vaccinate 70% of the global population against COVID-19. Pledging doses, however, is but a small step in providing equitable access to COVID-19 vaccines. More doses are necessary than what has been pledged by high-income countries, and many developing nations require the rights to make their own vaccines– a feat barred by intellectual property (IP) protection on coronavirus vaccines. Russia, China, and the United States have moved to support IP waivers for the vaccines, with support from other giants, including the United Kingdom and the European Union, still pending.
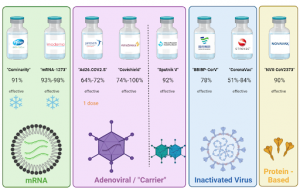
Though significant, overcoming the battle for intellectual property rights is but a comparably small obstacle for developing nations. The IP issue does not even account for concerns for manufacturing, distribution, and health infrastructure. The Pfizer-BioNTech and Moderna mRNA COVID-19 vaccines have become household names in the United States, although over 90 vaccines are in clinical trials. mRNA is incredibly fragile; therefore these vaccines require extremely low storage temperatures, even with the protection of the surrounding lipid nanoparticle. The sub-zero freezers necessary to store the mRNA vaccines would not be found in drug stores, doctors’ offices, most hospitals, and certainly not on a mountain in Cambodia. Pfizer’s vaccine, once thawed, only lasts in a refrigerator for 5 days, while Moderna’s vaccine lasts for 30 days. The logistics behind coordinating doses being thawed and administered also requires a sizeable amount of human resources, which is often another challenge for lower-income countries. In some circumstances, widespread distribution of these vaccines may not be feasible for many developing countries.
The Johnson & Johnson, Oxford-AstraZeneca, and Gamaleya vaccines are “carrier vaccines” which deliver the genetic code of the SARS-CoV-2 spike protein by way of a safe viral vector – an engineered adenovirus. Once the code is delivered to the cells, the cells produce a spike protein which trains the body’s immune system to make antibodies and memory cells to fight against the virus. The benefit of these vaccines is that they can be stored in a normal refrigerator for several months, making them more convenient to distribute. The caveat is that there are questions around the efficacy of these vaccines, especially against variants, though much has yet to be determined. Furthermore, the materials and human resources needed to manufacture carrier vaccines in-house pose more challenges to developing countries.
Various other COVID-19 vaccines have been developed including the protein-based vaccine by Novavax and the inactivated virus vaccines by Sinopharm and Sinovac. Each of these vaccines leverages different biological strategies to prepare our immune systems for battle. The vaccine response to COVID-19 is unprecedented, and there remain several unknowns about associated side-effects and long-term efficacy, among other factors.
Unfortunately, although there is no perfect, clear-cut solution or path towards providing equitable access to the COVID-19 vaccine at the moment, this pandemic has been and remains an important lesson in recognizing the ways in which science is interconnected with every aspect of society. We can celebrate new technologies and clinical advancements, but how much is it really worth without considering the larger picture of equitable access to these tools? I would like to believe that even if it seems like a world away, the Hill and the Mountain aren’t so distant — that even on opposite sides of the world, people can appreciate the equal value in humanity and therefore, the human right to health.
Peer Editor: Katelyn Huff