The year 2020 started out with a big scare due to the coronavirus outbreak, what is now officially known as Coronavirus Disease 2019 (COVID-19). There have been more than 75,000 COVID-19 cases globally and approximately 2,400 deaths, mostly in China, as of mid-February 2020. In the U.S, there have been 35 confirmed cases. However, this is not the first time that coronavirus is circulating in the human population.
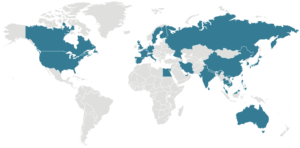 Green color indicates where COVID-19 cases have been confirmed as of mid-February 2020. Source: CDC
Green color indicates where COVID-19 cases have been confirmed as of mid-February 2020. Source: CDC
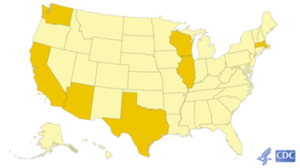 Dark yellow color indicates where COVD-19 cases have been confirmed as of mid-February 2020 in the U.S. Source: CDC
Dark yellow color indicates where COVD-19 cases have been confirmed as of mid-February 2020 in the U.S. Source: CDC
Common human coronaviruses have been constantly circulating within the human population and sometimes cause disease similar to the common cold. However, COVID-19 has spread at a speedy rate that leads to heightened global concern. Similar panic caused by coronaviruses has taken place in the past during the SARS-CoV outbreak in 2003 and the MERS-CoV outbreak in 2012. The common denominator for all of these cases is the ability of the coronaviruses to make the jump from an animal host to a human host, a process called zoonosis.
SARS-CoV was passed on from bats to civet cats to humans, and MERS-CoV was passed on from bat to camel to human. COVID-19 was found to be 88% similar to two bat-derived SARS-like coronaviruses. However, the intermediary animal between bat to human for COVID-19 is yet to be determined. So, how come COVID-19, SARS-CoV and MERS-CoV cause epidemics in human population but not in bats?
In a new study and an earlier report, researchers argue that the immune system of some bats, especially those who carry viruses known to be harmful in humans, allows them to carry a high load of viruses without getting sick. When viruses attack our body, our immune system will try to initiate inflammation. As this inflammatory response kicks in, we will feel a little (or a lot) lethargic and feverish. Not the best way to feel, but it is a sign that our body is actively attacking the virus. Bats, on the other hand, are able to balance between their robust antiviral response and anti-inflammatory response better than humans can.
The robust immune response coupled with very efficient anti-inflammatory response also allow bats to fly and live longer than most mammals at its size. As the only flying mammals, bats require high metabolic rates daily. Consequently, their bodies harbor high tissue damage and free radicals. If bats inflammatory response is as sensitive as humans, bats would not be able to live long. Thus, to compensate for these high loads of destructive molecules, they need to have a ‘dampened’ inflammatory response.
Scientists also showed in a computer model how infected bat cells release a warning signal, interferon-alpha, to other cells. This signal enables other cells to ward off the virus, but at the same time, the virus is still able to replicate without causing damage to the bat. The main concern is when these viruses make their way from bats to other animals and/or humans that do not have a similar immune response. Severe symptoms that potentially lead to fatal cases could ensue as our immune systems are overwhelmed.
As urbanization is more rampant, the community needs to be aware of disrupting bat natural habitats which may lead to more frequent cases of zoonosis. Scientists are continually trying to learn more about how virus transmission takes place between different hosts so that we can predict the emergence of future outbreaks.
Peer edited by Caitlyn Molloy
