What makes an antibody fabulous? To start, antibodies are complex proteins made by the immune system to protect the body from foreign invaders. Each antibody has unique “fingers” so that when it encounters a single unusual marker on a foreign invader that fits within its grasp, the antibody can swiftly grab onto the marker. Once attached, the antibody alerts other protective cells to engulf and eliminate the foreign invader which helps keep your body safe from diseases, viruses, and other unwanted molecules. Therefore, in each person there are over one trillion unique antibodies – at least one for every marker!
Most of the antibodies found throughout the body’s bloodstream are created before an infection and are known as natural antibodies. A smaller percent of antibodies, adaptive antibodies, are produced only after an infection, and are specifically built to attach to the invading molecule. During an infection, such as exposure to SARS-CoV-2, the body has an entire arsenal of immune processes and responses to call upon to protect itself including, but not limited to, producing adaptive antibodies.
Regardless if an antibody is natural or adaptive, each antibody shares the same symmetrical and characteristic Y-shape consisting of two longer heavy chains and two shorter light chains. The two identical heavy chains are shown in shades of pink and the two identical light chains are colored different shades of blue in the picture below. The left panel shows a real crystal structure of an antibody (known as “IHZH” on the Protein Data Bank site). The right panel shows a simplified cartoon representation often used to highlight the characteristic Y-shape.
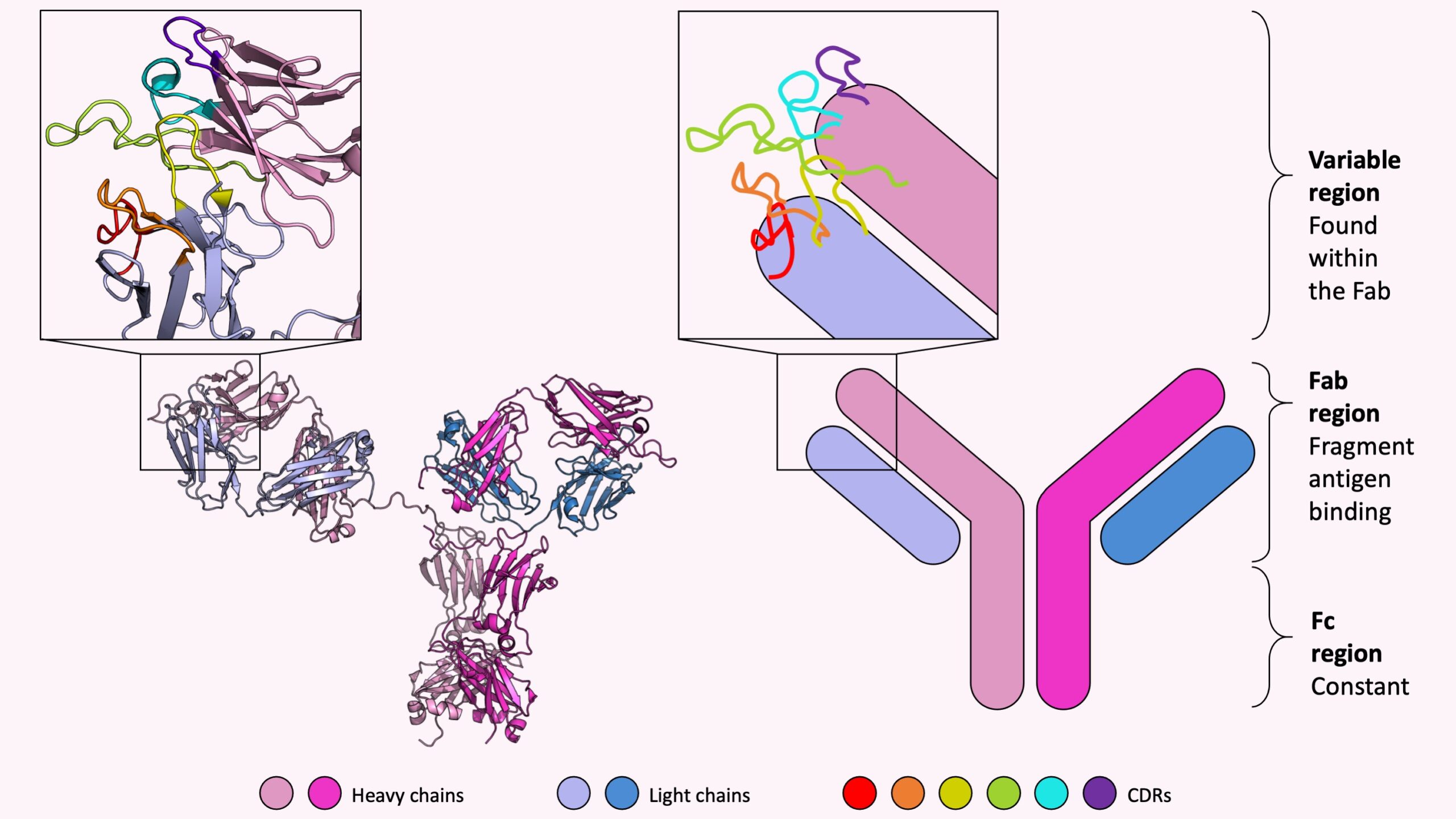
From these four chains alone, each human body is capable of making a quintillion, or a million trillion, unique antibodies – enough unique antibodies to defend the body from a variety of foreign invading molecules. Mathematically, how is that even possible? How can just four different chains be combined in over one trillion different ways?
The immense combination diversity comes from the different sequences of each heavy and light chain. More precisely, within the fragment antigen binding (Fab) region there is a variable region, which is found at the top right and top left end of each of the four chains in the above picture, that undergoes enormous sequence-level change to be tailored to attach itself to various unique molecules in the body.
Within the variable region there are six loops known as complementary determining regions (CDRs) which are long, flexible, easily modified, and responsible for binding the unwanted molecules; these loops are like fingers on a hand which delicately sway and search for unusual molecules. Once the CDR loops recognize the unusual foreign molecule they were designed for, then the fingers grab on tightly to the molecule then rigidly hold it in place. The picture above shows a zoomed in view of the six CDRs colored red to purple on the end of one side of an antibody, for both the antibody crystal structure and the corresponding cartoon diagram.
So what part of an antibody makes antibodies fabulous? The answer is in the name itself! The Fab region holds the majority of sequence diversity ensuring that each antibody is unique and specific for each of the various molecules found in the body – which sounds pretty fab(ulous) to me.
Peer edited by Sean Gay
This is an extremely interesting article for a lay person with the beginnings of dementia. I am amazed at the number of antibodies within each individual, a million trillion. And the binding process which attaches to unwanted molecules, like in an illness, is like WOW! The work done by the young scientist in this study, birthing the FAB Four, is truly amazing!