Antibiotic or drug resistance occurs when bacteria or fungi are no longer impacted by medicines designed to inhibit their growth or kill them. The emergence of drug resistant bacteria and fungi is a growing problem around the world and can lead to increased difficulty in treating infections, increased medical costs, longer hospital stays and increased mortality. The Centers for Disease Control estimates there are approximately 2.8 million infections with drug-resistant bacteria and fungi each year. A recent report of two outbreaks of a drug-resistant fungus in health care facilities in Texas and the District of Columbia between January and April 2021 suggests the problem may be getting worse. The most obvious solution is to develop new drugs for which there is no resistance. However, the biology of developing new drugs, particularly against fungi, is tricky.
What is fungus?
Fungi are a diverse group of organisms including yeasts, molds, and mushrooms. The different fungal species can range in size from the large honey fungus, which spans 2.4 miles across to the microscopic brewers and bakers yeast used to make beer and bread. Fungi are found everywhere and anywhere that can sustain life. In fact, you probably have some growing in your fridge or shower right now.
We come in contact with fungi every day and anyone can get a fungal infection, but infections occur most often in people with a weakened immune system. Fungi can cause a variety of diseases and infect almost every organ in the body. Some people are familiar with the less severe oral thrush or ringworm. However, more invasive infections of the lungs, brain, or blood can be life threatening. Currently, the treatment options for fungal infections are sparse. There are only three groups of drugs that can be used to treat fungal infections and not all species of fungi are killed by all three groups of antifungal drugs. Further, the spread of antifungal resistance is making it difficult to even treat infections caused by fungi that would normally be susceptible to killing with antifungal drugs. In some instances, including some of the recent cases in Texas and the District of Columbia, fungal infections are resistant to all known antifungal drugs. Patients with fungi resistant to all known antifungal drugs can be treated with other medicines. However, these medicines may be less effective and come with more severe side effects, an important factor considering that many patients with drug resistant infections are already ill from an underlying condition or procedure.
How scientists engineer new antifungal agents
The world clearly needs new drugs to treat fungal infections, so why can’t scientists just develop new drugs? Developing new drugs to treat bacterial and fungal infections is a long and painstaking process. Scientists generally have two options for drug development. The first is to identify part of the bacterium or fungus to target with a new drug specifically designed to change that piece. The second is to test many drugs (sometimes over ten thousand) against different bacteria or fungi to see if any of the drugs kill the bacteria or fungi. The component of the fungus that is targeted by the drug is then identified.
While these processes are arduous, developing drugs to work against fungi presents a unique problem. When developing a new drug, you want a drug that specifically kills the bacteria or fungi without affecting the human cells. To do this, you need to identify a component that is found only in the bacterial or fungal cells, but not in human cells. Bacteria are very different from human cells giving scientists many unique parts to target with drugs without affecting human cells. However, fungal cells are much more similar to human cells than bacteria and are even made out of some of the same components. This means there are many compounds that kill both fungal cells and human cells and there are only a couple of parts of the fungi that can be used as potential drug targets.
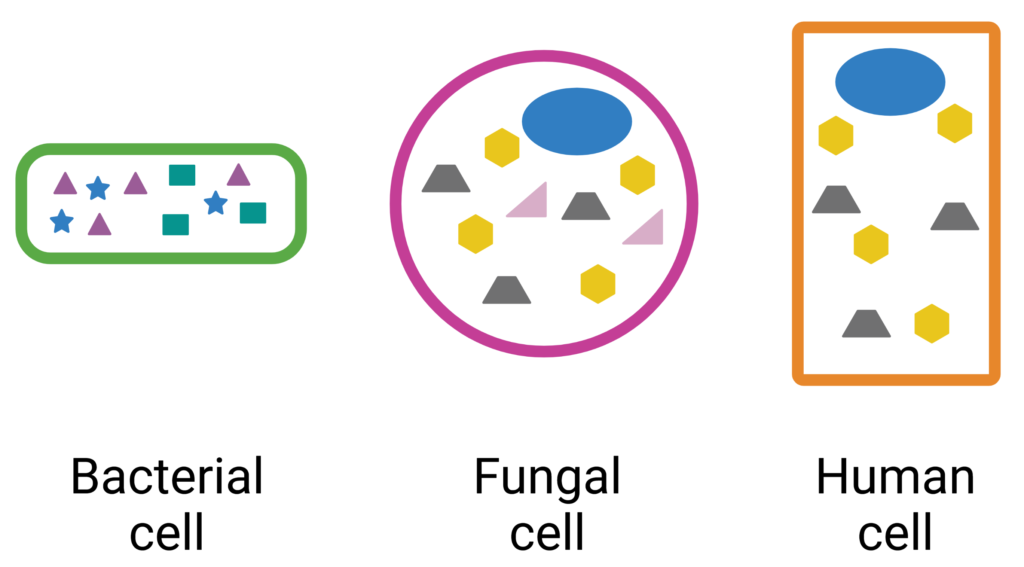
For example, if we take the the cells depicted in Fig 1, we see that there are many differences between the bacterial cell (far left) and human cell (far right) (i.e. green outline, the purple triangles, blue stars, or the teal squares). Therefore, a drug that modifies any of these components that are unique to the bacterial cell would likely have little to no effect on the human cell. However, when we compare the fungal cell (middle) to the human cell, we see that the only components that are unique to the fungal cell are the pink outline and the pink triangles. Drugs could modify the pink outline or the pink triangles without impacting the human cell, but drugs that modify the yellow hexagons, gray trapezoids or blue circles would likely kill the human cell, as well as the fungal cell.
Are there any solutions?
The need for new drugs to treat fungal infections is made more dire by the emergence and spread of drug-resistant fungi. In recent years, scientists have made headway in developing treatments for fungal infections. Multiple drugs are currently in clinical trials and show promising results in the treatment of fungal infections. Notably, a new antifungal treatment was just approved by the Food and Drug Administration in June 2021. The drug, ibrexafungerp, inhibits an enzyme in fungi used to construct the cell wall, a component of fungal cells that is essential for survival. Although Ibrexafungerp is currently only approved to treat vulvovaginal candidiasis, clinical trials examining its use in treating other types of fungal infections including invasive infections are underway. Most excitingly, ibrexafungerp represents a new type of antifungal that could potentially be used to treat drug resistant fungal infections. With any luck, this will be the first of many.
Peer editor: Odessa Goudy