This past October, CBS 60 Minutes aired a feature on Artificial Intelligence. They were taking a peek into the world of Watson, a computer system developed by I.B.M that can answer questions framed in natural languages. The same 60 Minutes episode also featured Dr. Norman Sharpless, Director of the UNC Lineberger Comprehensive Cancer Center, who provided an overview of how Watson’s artificial intelligence is being used at UNC to make treatment decisions for cancer patients.
Watson became an instant celebrity in 2011 when it was successful in beating human champions of the quiz show ‘Jeopardy!’. Since then the ‘robo-geek’ has come a long way, and its ability to analyze and interpret information has taken a big leap, so much so that it can now read and keep track of thousands of academic papers, medical records and clinical trial results, and then translate that information to make potential treatment suggestions to clinicians.
In 2015, I.B.M. began collaborating with pioneering cancer institutes across the nation, including UNC Lineberger, to hasten the process of analyzing the patient’s cancer on a molecular level. By exploiting Watson’s data analysis and data visualization capabilities, I.B.M and UNC seek to tailor suitable treatment options. With the special segment on 60 Minutes, UNC got a fair share of spotlight in primetime national television. But, is crazy to use a computing device to make critical treatment decisions in a disease like cancer?
Every living organism, including humans, are composed of millions of cells, the basic fundamental units of life. Cells contain DNA, which encodes the necessary instructions required for a living organism to survive and grow. These instructions are found in segments called genes. During exposure to environmental factors like sunlight, smoking or ageing, the DNA may become damaged. While the cells have their own tools to repair damaged DNA, sometimes the damage is too overwhelming and may escape the cell’s quality control mechanisms. The damaged DNA, also known as mutated DNA, may be passed onto subsequent generations of cells. The mutant DNA may now instruct the cells to grow uncontrollably, and as a result the cells can deviate from their normal functions. This is when a cell can turn cancerous. When a group of these cancerous cells clump together, they form a tumor. Historically, cancer treatment regimens have been directed toward where the tumor is located in the body, and while this has some positive therapeutic effects, it has not been very efficient.
Over the years, as genetic research progressed, scientists began to classify tumors on the basis of their genetic characteristics. They are now able to identify the genes and the corresponding mutations that drive tumor growth. Drugs targeting these mutations have been designed, and some of them have demonstrated considerable improvement in patient survival. The question now is can cancer be effectively treated based on the broad pathology of the tumor in the body. An individualized approach based on the genetic makeup of the patient’s cancer is being considered as an alternative treatment.
If this sounds too abstract, let’s consider a clinical example. It is well established now that tumors in some breast cancer patients have high expression of estrogen receptors. This means that the cancer cells may receive signal from the hormone estrogen, which may facilitate tumor growth. A class of drugs called ‘aromatase inhibitors’ prevent the body from producing estrogen and may work well on this subset of patients. However, only about half of patients whose tumors tested positive for estrogen receptors responded to these class of drugs. With a genetic-based approach to cancer treatment, the genetic profile of the patients who respond to the therapy may be used to look for treatment patterns. Now, if these patterns are consistent with one another and they match the genetic map in a section of patients that respond positively to aromatase inhibitor therapy, then this may stand as an effective treatment strategy. Other estrogen receptor positive patients may be treated using a different strategy without wasting time on a therapy that may not work. This new era of treatment is referred to as the age of ‘precision medicine’.
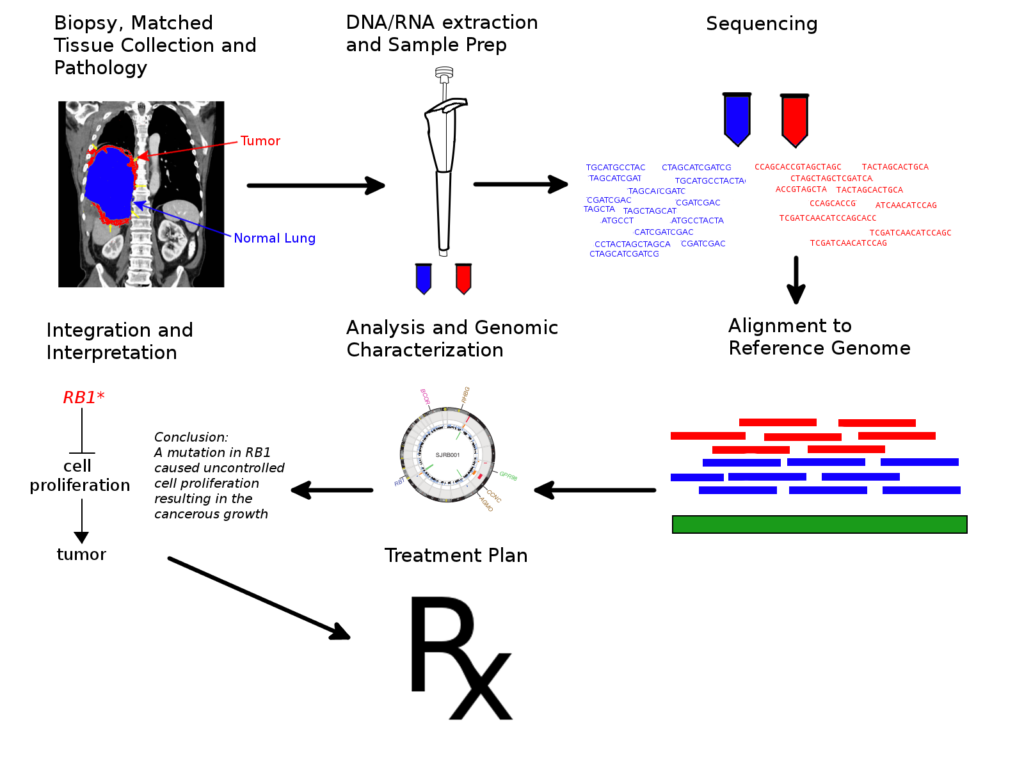
At UNC, when a patient agrees to participate in the ongoing clinical trial UNCseq, a tissue sample is collected by biopsy or during surgery and is analyzed to determine the genetic makeup of the tumor. A blood sample is also drawn to examine the genetic profile of the patient. The samples are then analyzed by rapid DNA sequencing methods. Then the building blocks of the cancerous and normal DNA are compared one block at a time to identify the mutations present in the tumor. Now, if a mutation affects a protein which helps a cell to survive and grow normally, it may be a cancer-driving mutation. However, there may be hundreds to tens of thousands of other ‘innocent’ mutations that do not affect cancer growth. Rigorous analysis is required before key mutations can be determined.
A single patient’s genetic information may clog up gigabytes of storage space, and analyzing all of these data to search for patterns may require a fair amount of mathematical effort and computation time. All of this occurs in a situation where a patient awaits treatment, so every bit of time is precious. This is where a supercomputer like Watson comes in. Watson had been able to analyze the DNA profile of tumors very efficiently and proposed treatment regimens that matched the decision of the clinicians 99 out of 100 times. What is more interesting is that in about one third of the cases, it was able to identify new treatment strategies that the doctors had not previously considered. It is to be seen whether this approach helps improve overall standards of care in a large cohort of patients in the long haul.
Cancer therapy still has a long way to go. Just because we know about the aberrations in the cancer cell doesn’t mean we can develop a complete cure. Current chemotherapy drugs can effectively kill cancer cells, but they may pose significant toxicity to the patient as they also attack healthy cells. Targeted therapies against genetic mutations work very efficiently in a few cancers, but only for a short span of time. The cancer becomes resistant in days to months and returns aggressively. Immunotherapy approaches where the immune system is stimulated to fight cancer is the new hot-topic in clinical discussions. Unfortunately, only a small subset of patients respond to these therapies, and for unknown reasons. However, with precision medicine, we expect to provide the most appropriate care to each patient, resulting in considerable improvements to their survival and quality of life. We continue our fight against beating cancer, and as a Tar Heel, I am proud that UNC is leading the way.
Edited by Nicole Tackmann and Alison Earley.
Follow us on social media and never miss an article: