It is said that the fastest way to someone’s heart is through their stomach. Sequencing, the technology that allows us to read the genes present in a sample, is important for understanding the health and disease of the heart. Although this sounds like a complicated approach to some complex biology, we can reframe sequencing as a gastronomic adventure to better understand the biology of the heart. The heart is made up of several unique cells. Each type has a unique identity and function that makes the sum of its parts greater than the whole. To understand the importance of each component, or cell type, we can mash tissue up and investigate the samples we take.
RNA is an important intermediate molecule between DNA, a set of genetic instructions, and protein, large complexes made from those instructions. RNA provides information on genes that are being expressed – what actually gets made by the cell. The traditional method of bulk RNA sequencing can be thought of like a smoothie, in which different fruits are blended together to make a homogeneous mixture. You sip the homogenate to sample the combination and can pick up the presence of blueberry and banana, but it’s difficult to pick up the hidden presence of sneaky spinach. Sampling this mixture, it is also nearly impossible to determine the exact ratios of each ingredient. Similarly, with bulk RNA sequencing, many different cell types from tissue are broken down and mixed together to obtain RNA. Our heart “smoothie” measures the average expression of genes from all the cells combined. We will not be able to pick up rare cell populations that get drowned out by the contributing blueberry and banana cells, nor do we know exactly how many blueberries are present in the sample. In the context of the biology of the heart, cardiomyocytes which contract and allow the heart to beat, are like the banana in the smoothie as they make up 70% to 85% of the volume of the mammalian heart. Endothelial cells, which line blood vessels, are more like spinach as they only make up about 8% of the cells in the ventricles.
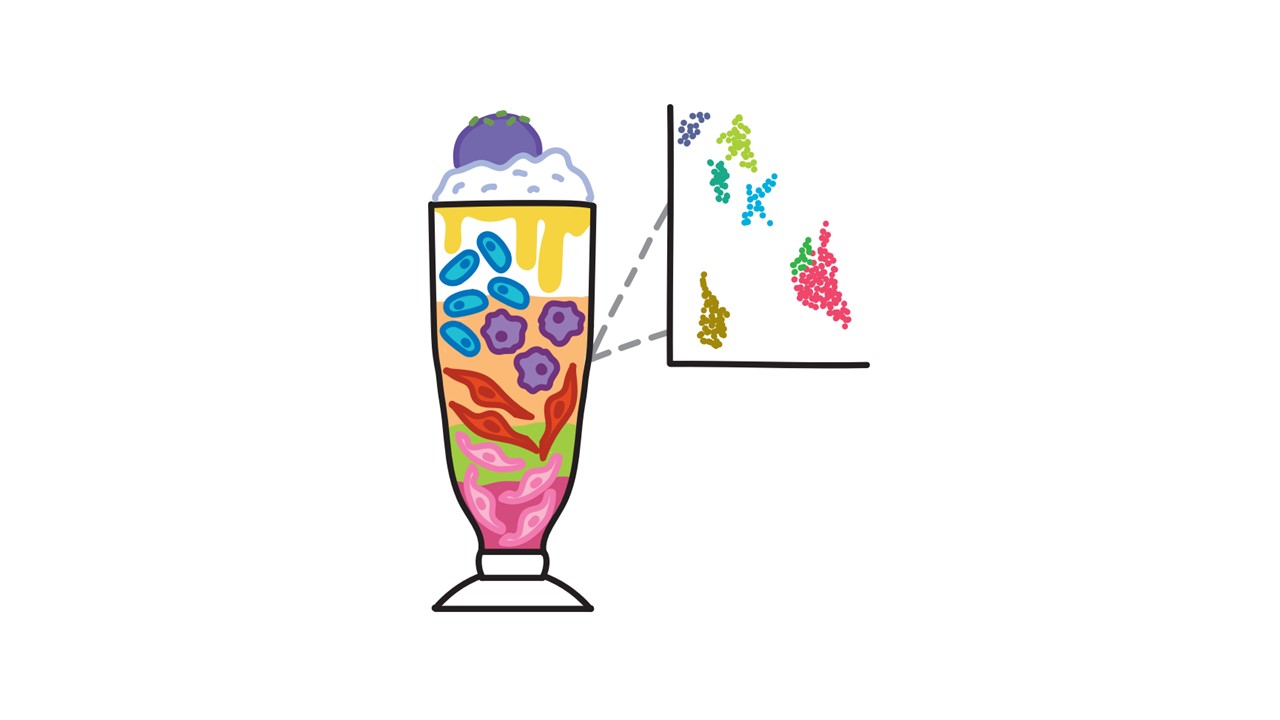
Over the past couple of decades, a high-resolution sequencing technology, single-cell RNA sequencing (scRNA-seq) has been developed and used to study gene expression at the level of individual cells. Instead of a smoothie, this method can be compared to a culinary treasure of the Philippines called halo-halo, which translates to “mix-mix”. This dessert combines sweet beans, tapioca balls, coconut strips, softened yam cubes, a version of Rice Krispies called pinipig, and sweet ube yam paste over shaved ice and condensed milk. It is customary to gently mix all the ingredients together in a tall glass before taking a spoonful to sample the combination. Unlike the smoothie, halo-halo allows us to distinguish every single ingredient – including the hint of puffed rice, and how many beans versus yam cubes are present. In the heart, there is a small number of endothelial cells in healthy physiologic conditions. In an unhealthy pathologic state, a fraction of those cells become diseased endothelial cells. The scRNA-sequencing method provides us with a high-resolution approach that can tell us exactly how many healthy and unhealthy endothelial cells are present in our sample. Therefore, scRNA-seq data allows us to identify and quantify these rare cell populations, as we know the gene expression profile of each individual cell.
So, why entrust your heart to halo-halo over a smoothie? As previously mentioned, each cell has a unique identity and function. ScRNA-seq gives a more definite picture of which cells are present and the genes being expressed; an important tool when comparing states of physiology and disease. In a sample from a subject with cardiovascular disease, it would be possible to identify cell populations unique to the disease state that are not present in a healthy physiological state. Furthermore, by understanding the genes expressed by each and every cell it becomes possible to map how cells change from a healthy to a diseased state. More recently, scRNA-seq has been applied to the study of atherosclerosis – a thickening and hardening of arteries caused by the accumulation of lipid deposits and plaque. This process involves various cell types including smooth muscle cells, macrophages, and endothelial cells. Regions of the coronary arteries that experience less regular pressurized blood flow are more susceptible to lipid deposition and plaque build-up. Over time, smooth muscle cells collect within the vessel, causing a thickening of the wall. Immune cells like macrophages then migrate to these regions and contribute to inflammation and build-up of lipid deposits. Eventually, these regions develop into lesions and harden into a calcified state of variable stability. When the plaque is unstable and ruptures, it can result in a heart attack or stroke. ScRNA-seq allows us to capture information on every single cell involved and how they change over the course of this process. When compared to a control state of physiology, we can identify changes that occur with disease. More importantly, this method allows us to answer specific and significant questions about how the cells may be changing and by what mechanisms.
At the end of the day, the smoothie and halo-halo approaches each have their strengths and trade-offs. In bulk RNA-sequencing, we get an average of expression and can broadly identify the differences between at least two conditions. It is relatively straightforward to mash up whole tissue and prepare the sample for sequencing at a fraction of the cost of scRNA-sequencing. On the other hand, scRNA-sequencing provides an unprecedented resolution of gene expression allowing for comparisons across individual cells studied under at least two different conditions. High-level bioinformatic analysis can be performed on these data sets to gain meaningful insights, however, the learning curve and exceptionally high cost of scRNA-sequencing limits its applicability and scalability. Therefore, deciding which treat you will splurge on depends on the questions being asked.
Peer editor: Caitlyn Molloy