We’ve all heard of the CRISPR/Cas9 system – a genetic engineering tool that has revolutionized the field of biology through genome editing. We’ve also heard of genetically modified organisms (GMOs) in the context of modified crops that have higher yield, meatier cows that produce more beef, or even fluorescent pigs. What if we were able to extend the reach of genome editing by putting these two aspects of genetic engineering together to then modify whole populations and species in just a few generations? Welcome to gene drive!
Sexually reproducing organisms have two copies of most genes – one on the chromosome from the male parent, and one on the chromosome from the female parent. Genes also have alternative forms (called alleles) that are found at the same place on either chromosome, but can produce different phenotypic outcomes. An individual that has two chromosomes with the same allele is said to be homozygous for it, while an individual that has two chromosomes with different alleles is said to be heterozygous for it. Under regular Mendelian inheritance, the probability of inheriting a particular allele from a heterozygous parent is 50% – this is because offspring can only inherit one or the other chromosome from each parent. However, gene drive is a technology that changes this probability and makes it much higher than 50%. Theoretically, gene drive makes it possible to have a certain allele be inherited 100% of the time!
How does this work? Well, one of the most powerful gene drive mechanisms involves the use of the CRISPR/Cas9 system. In the germline of an organism heterozygous for the drive allele (the allele that we are trying to propagate through the population), Cas9, a protein that can cut DNA, creates a double strand break (DSB) on the chromosome that doesn’t have it. This prompts the cell to repair the DSB by copying in the drive allele from the homologous chromosome, leading to the cell becoming homozygous for the drive. Every meiotic product – or gamete – produced will now have this allele, ensuring that it is passed onto every offspring!
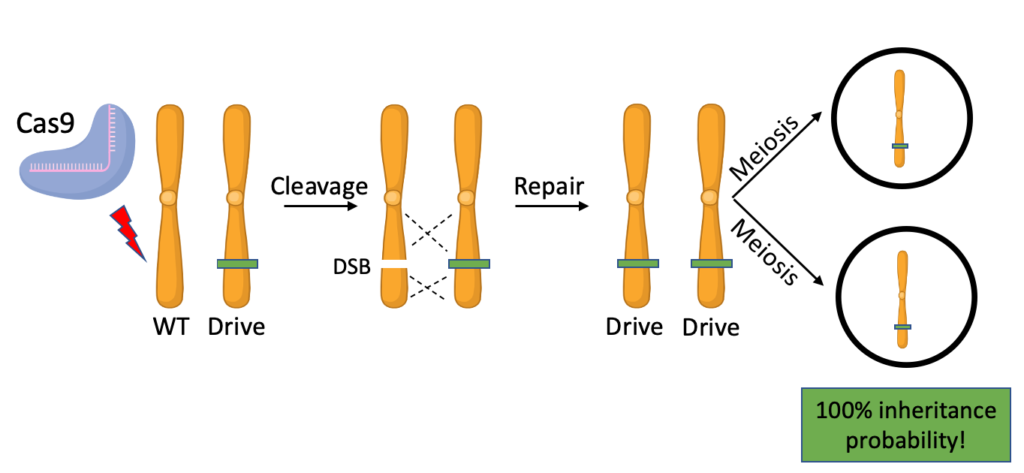
However, this may lead you to question how subsequent generations of progeny are guaranteed to inherit the drive. If the gametes produced by the first generation of offspring are fertilized by individuals without a drive allele, the inheritance probability of this allele will drop back down to 50%, which is not sufficient to spread it through a whole population. To make the system self-propagating, Cas9 needs to make a DSB on the wild-type chromosome and copy in the drive allele in every generation. With all the pieces of the puzzle already laid out on the floor, this is done easily enough: all we need is for the drive construct to include genes coding for the CRISPR/Cas9 system within it. This gives the drive construct the power to cut, copy, and convert a wild-type allele into the drive allele every time it’s inherited. Therefore every individual that inherits the drive will pass it on no matter what allele it gets from the other parent!
CRISPR based gene drive systems have been proposed as a solution to vector borne diseases, pest invasions, and even certain viral infections. This is possible by propagating a set of genes that either kill, or reduce the pathogen carrying efficiency, of insects such as mosquitoes that can spread malaria, dengue, zika, etc. Gene drive can be used to lower fertility, skew sex ratios, and even reduce infectivity of the populations it targets. However, it is important to mention that while gene drive systems may prove critical in the fight for better public health, the concept of genome modifications at the population level brings with it many ethical concerns. It has never been easier to wipe out entire species with little to no knowledge of how local ecosystems would be affected. Furthermore, genome modification as it relates to human gene editing is always a worrisome subject. Add to that the population level scale at which gene drive functions, and we can’t help but wonder: are we inching closer to alarming bioethical grey areas? What would the world look like if an extinction-based drive system found its way into a human embryo?

Peer edited by Jamshaid Shahir