Introducing a Circular Energy Economy
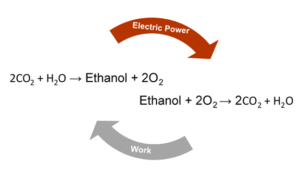
In this age, revolution is growing, and in many ways, our future is improving from the efforts of millions. As climate change continues to acidify our oceans, intensify weather, manipulate seasonal cycles, and threaten coasts, the use of CO2 producing fuel sources needs to be harshly reduced if not eliminated. By converting CO2 into new carbon molecules, we can become carbon neutral at the least and generate a circular energy economy. A circular energy economy is one in which all the chemical inputs can be obtained from the output of the cycle. What this looks like is the input of CO2 and water producing ethanol, and the burning of ethanol in oxygen leads to release of CO2 and water. A circular energy economy still requires external energy to produce the ethanol fuel source, but in return, ethanol production could be used to store transient forms of electricity such as solar, wind, and current-based sources of electricity. This storage may seem trivial at first. After all, we have rechargeable batteries, but a one-pound lithium ion battery can only store about 5% of what could be extracted from a pound of ethanol. To make transient electricity sources the dominant supply, there needs to be a way to store that electricity efficiently. Ethanol provides a great storage strategy due to its low toxicity and high energy density as well as the fact that it is currently incorporated into fuels for combustion engines. Ethanol would still be able to return electricity to the grid as well through devices called fuel cells. Additionally, the manipulation of CO2 using sunlight is reminiscent of photosynthesis, and similar to how researchers have made more efficient chloroplasts, the future of energy and chemical feedstocks may be an improved simulation of a plant’s growth.
Recent Marvels in CO2 Reduction
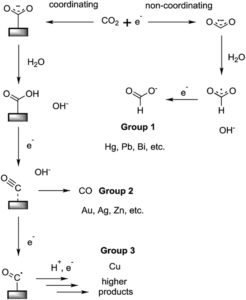
Each day, humans produce roughly a kilo of carbon dioxide from just our breath. If this CO2 were to be transformed into ethanol completely, that would be 0.7 liters of ethanol produced. Ethanol is the two carbon alcohol that humans prepare in booze and one of the only nontoxic alcohols. Given the current leading CO2 reduction technology, the yield of ethanol is about 50% relative to the electricity and CO2 that are driven into the system, so at the moment, we would be able to extract about 0.3 liters of ethanol from collected human breath daily. There are a couple reasons I find this fascinating. First, as a chemist, it amazes me that in about three days, I could collect enough CO2 to replicate vodka with my breath and some good electrochemistry. The law of conservation of mass could be constantly evidenced by my overworked still and electrodes. Secondly, 50% conversion efficiency for CO2 to ethanol makes a circular energy economy seem feasible within my lifetime, and it also represents a significant achievement in the field. As can be seen from the image below, a variety of chemicals can be produced from CO2 reduction depending on which surface is used in the reduction device. The single carbon product made by Group 1, known as formate, has a low utility and predominantly is only used for deicing of airplanes, and while carbon monoxide (CO) made by Group 2 has use as a synthetic chemical and can be used for energy storage, it’s both toxic to humans and much more difficult to store and transport than ethanol. By optimizing the yields of ethanol from CO2, a fuel source with constant demand can be made from a greenhouse gas. Unlike formate, ethanol is employed in a variety of applications, so the demand should be able to support CO2 reduction’s economic deployment. Since ethanol is not exclusively used as a fuel, a net decrease in CO2 concentration could be realized by an active market generated by the novel CO2 reduction we can see today.
Climate Change Can Be Curbed, If We Try
As the chemistry surrounding electrochemical CO2 manipulation expands, valuable materials for chemical synthesis and consumer products once derived from petroleum may be derived from the very air we exhale. Humanity’s science is improving every day, and I believe that soon the United States and many other nations will be able to use fossil fuels as the supplement to our energy needs and sustainable energy sources will dominate, but that means that funding of science and renewable energies must persist until it becomes the self-sustained economy that I’ve presented here. That means our governments must be full of informed and concerned representatives who want to curb climate change at every level, and that means that every person around the globe must make addressing climate change a goal they consistently pursue in their investments of energy, capital, and electoral power.
Peer Edited by FanTing Kung