What started as a pleasant day on the beach turned into a near death experience for Australian man Jaun-Paul earlier this year. While enjoying a routine dip in the water, he encountered a small, but deadly, blue-ringed octopus. Blue-ringed octopuses are usually no bigger than 20 centimeters long, nor heavier than a paltry 100 grams, but they pack a wicked punch in their bite that often feels like nothing more than a bee-string, if it hurts at all. A bite from a blue-ringed octopus can lead to paralysis within less than an hour. Paralysis of respiratory muscles can lead to hypoxia and, though quite rare with the appropriate care, death.
Jaun-Paul was lucky enough to be rushed to a hospital right away for lifesaving care. The report by 9 News Australia revealed the general consensus about blue-ring octopus envenomation: locals think that it’s a death sentence. It turns out that the fast acting poison, tetrodotoxin (TTX), has no antivenom.
It was surprising to me that an unimposing, somewhat cute creature such as the blue-ringed octopus could be so dangerous. However, they aren’t the only small and friendly-looking sea creature whose venom has the potential to kill. TTX is also used by other marine animals such as pufferfish and some types of newts and starfish.
To get an idea for why TTX poisoning is not yet directly treatable with an antivenom, one should take a closer look at how it actually works in the body. TTX works by blocking our voltage-gated sodium channel proteins (Na channel proteins for short). These proteins allow charged particles to cross through our cell membranes, resulting in a voltage difference that excites our cells. This process is what allows muscle cells to contract, endocrine cells to release hormones, and neurons to send signals to and from our brains. Because TTX blocks Na channels that are primarily located in the central nervous system, TTX is classified as a neurotoxin and as such, can ultimately result in deadly paralysis.
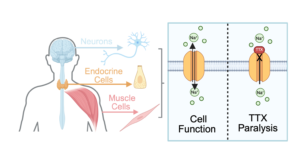
Since we know how it works, why can’t we create an antidote? The reality of the mechanism may just be the answer.
One way to deal with a molecule that binds a protein you don’t want it to is to make another one that binds even better to displace it, perhaps allosterically (i.e., in a way that changes the shape or activity of the protein). However, TTX does too good a job at blocking our sodium channel proteins: it binds very tightly, and takes a very long time to dissociate, such that it would be very difficult to design something that can compete. And consider the flip side of coming up with such a molecule – it could very well exacerbate the problem! Something that blocks our sodium channels even better than TTX would just paralyze us faster.
Another more suitable approach for a poison like TTX would be to encourage our bodies to break it down faster. However, our bodies don’t produce enzymes that are capable of digesting TTX in the first place. We have tried to opsonize (ie, flag foreign objects for breakdown by attaching antibodies or other proteins to them) TTX, but this has had limited success. TTX needs to be unbound to be effectively opsonized; it could be that it binds to our sodium channels so quickly that nothing else can get to it in time. Antibodies have shown protective effects in mouse studies, but they have to be administered before TTX exposure.
Although there is no antidote for TTX, contrary to what the locals interviewed by 9News Au may think, most people who are exposed don’t die. This is because we can perform dialysis to help flush TTX out of the body, as well as use a ventilator in the event of respiratory paralysis. TTX has limited effect on the brain and heart, so there typically aren’t lasting effects.
Despite the lethality of TTX, we may have found some use for it in the clinic. Recall that TTX can block the signals neurons send to and from the brain. This feature makes it attractive in pain management therapy, as pain signal generation and propagation can be blocked in the central nervous system using (far) less-than-lethal doses of TTX.
Next time you are out enjoying a day on the beach, especially by the warm, shallow waters off the coasts of Australia or Japan, keep an eye out for those small, cute, and not-so-friendly sea creatures. Jaun-Paul, luckily, made a full recovery after being rushed to the hospital. If you get stung or bitten, look out for the signs of TTX poisoning and act quickly!
Edited by: Sarah Lester
Illustrated by: Katie Holmes