While you’re reading this, millions of immune cells in our body are having the most important conversation of the day.
The Group Chat That Never Sleeps
Our immune system is less like a collection of individual security guards and more like a massive group chat that’s always active. Immune cells are constantly communicating through chemical signals called cytokines (think: text messages), chemokines (location pings that attract cells to specific locations), and even direct cell-to-cell contact (the equivalent of pulling someone aside for a face-to-face conversation).
T cells, B cells, macrophages, dendritic cells: they all have specialized roles, but none of them can do their job alone. T cells are like your extroverted friend who organizes game night, coordinating the response. B cells are the friend who is always taking pictures; they never forget a pathogen you have encountered. Macrophages are the multitaskers, simultaneously attacking threats while sending updates to the rest of the team.
The coordination is fascinating. When you get a cut, immune cells don’t just randomly show up; they recruit each other in waves, with precise timing. First responders send signals that call in backup. Some cells create inflammatory signals to sound the alarm, while others (like regulatory T cells, or T-regs) send calming signals when it’s time to stand down.
It’s not just about having the right cells show up to the party; it’s about whether they’re actually talking to each other.
And it turns out, our immune cells are the most social beings in our body.
This October, the Nobel Prize in Physiology or Medicine went to three scientists who fundamentally changed how we understand immunity. Mary Brunkow, Fred Ramsdell, and Shimon Sakaguchi discovered regulatory T cells. These are specialized immune cells that act as “security guards,” preventing the immune system from attacking our own tissues. But here’s what makes their discovery so profound: it wasn’t just about finding new immune cells. It was about understanding how immune cells coordinate with each other.
When the Telephone Game Goes Wrong
Now imagine that group chat starts breaking down. Messages get misread. Some cells stop responding. Others start spamming the chat with mixed signals. This is increasingly what we think is happening in a startling range of diseases.
In cancer, regulatory T cells can infiltrate tumors and suppress the immune system’s ability to mount an effective attack. The immune cells are there, but the tumor microenvironment disrupts their coordination, allowing tumors to evade detection. In autoimmune diseases, immune cells misread signals and attack the body’s own tissues, a phenomenon that disproportionately affects women, as we’ve previously covered on the Pipette Pen. In neurodegenerative diseases like Alzheimer’s and ALS, we are finding that immune coordination both in the brain and throughout the body becomes disrupted: peripheral immune cells may infiltrate the brain, microglia (the brain’s primary immune cells) may send the wrong inflammatory signals, and the normal crosstalk between the central nervous system and the peripheral immune system breaks down. Their communication network is in chaos.
This realization is changing how we think about disease. It’s not always that we have too many or too few immune cells. Sometimes the problem is that they’re simply not coordinating properly.
Fixing the Conversation
If miscommunication is the problem, maybe we can fix the conversation?
Several breakthroughs in immunotherapy have emerged from understanding immune cell communication. Immune checkpoint blockade therapies like anti-PD-1 and anti-CTLA-4 antibodies—essentially “unmute” T cells, removing the inhibitory signals that cancer cells exploit to suppress immune attacks. These therapies have revolutionized cancer treatment by restoring the immune system’s ability to coordinate an effective response. Another breakthrough is CAR-T cell therapy, which engineers a patient’s own T cells to recognize cancer cells directly. It’s like giving immune cells a new phone number to bypass the tumor’s attempts to disrupt normal communication.
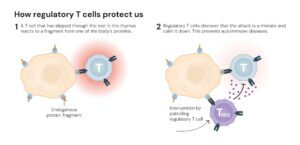
The discovery of regulatory T cells and their control, this year’s Nobel Prize-winning work, has also opened new therapeutic avenues. Over 200 clinical trials are currently investigating regulatory T cell-based therapies for autoimmune conditions, transplant rejection, and chronic inflammatory diseases. There’s even research into whether we can modulate immune cell communication in aging and neurodegeneration to reduce harmful inflammation while preserving protective responses.
We’re still in early days, but these studies are promising: instead of just suppressing or boosting immunity, we may be able to restore communication between immune cells.
The Conversation that Keeps Us Alive
This year’s Nobel Prize recognized something profound: the immune system’s power comes not from brute force, but from coordination. Our immune cells are like social beings, and their ability to communicate is what keeps us healthy.
So the next time you fight off a cold or heal from a scrape, remember: it’s not just your immune cells showing up. It’s millions of them having a sophisticated conversation to coordinate a real-time response and keep you alive.
Peer edited by: Katie Holmes