As he was dropping frogs into giant magnets in 1997, physicist Andre Geim probably didn’t expect that his research would result in anything earth-shattering. His brilliantly titled paper “Of flying frogs and levitrons” would land him an Ig Nobel Prize, an annual award devoted to “achievements that make people laugh, and then think.” But thirteen years later, Geim found himself onstage in Sweden accepting the 2010 Nobel Prize in Physics for his “groundbreaking experiments regarding the two-dimensional material graphene,” and over the next six years, publications about graphene more than tripled.
But recently, public and academic interest in graphene has plateaued. Why might that be? To answer this, it would probably be worth seeing what exactly graphene is, why it sparked so much curiosity, and what its limitations have turned out to be.
As your organic chemistry classes may have taught you, carbon is an extremely versatile element. It bonds easily with itself and other elements, so it comes in many interesting forms called allotropes (like diamonds and graphite). Graphene is a 2-dimensional allotrope of carbon with a simple structure: an atomically flat honeycomb pattern, where the corners of each hexagonal unit have just a single carbon atom, and the edges are bonds between the atoms. One exciting consequence of this 2D structure is an extremely high mobility, such that electrons in graphene move crazy fast. In other words, electricity moves very quickly through graphene. By comparison, silicon, the element we use in circuits for computers, has a mobility just one tenth of graphene’s.
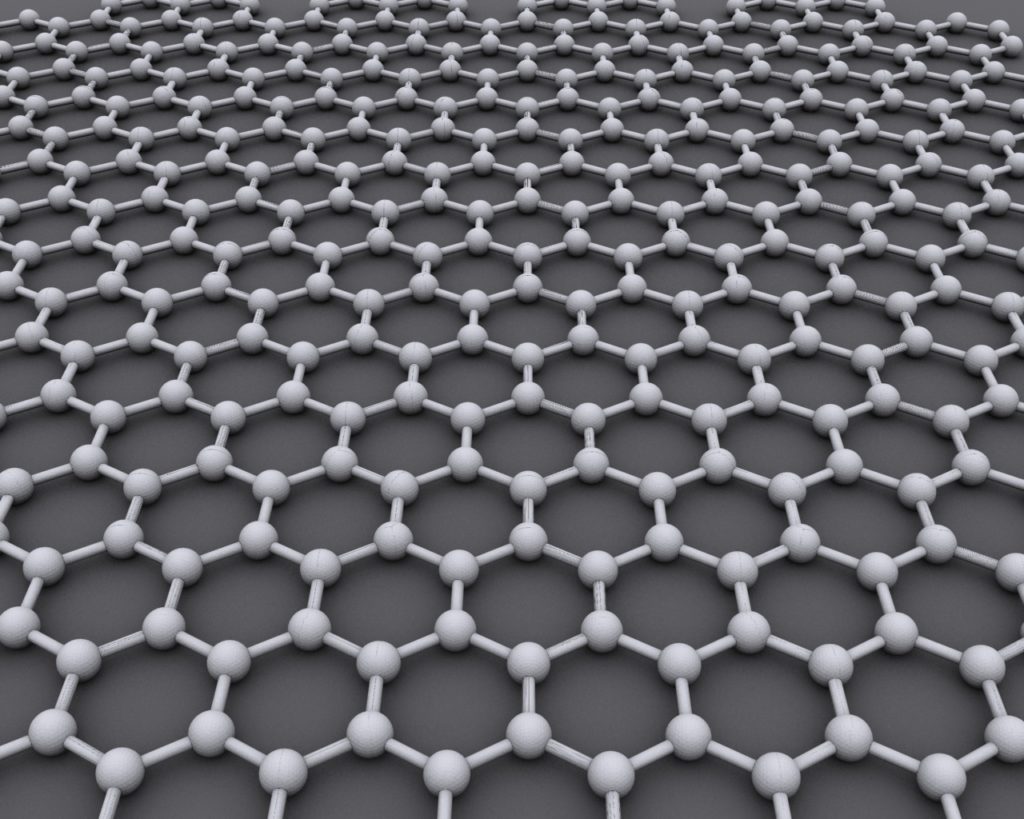
This and other fascinating electrical properties floored academics and laypeople alike, and left just about everyone scrambling in what was dubbed the “graphene gold rush.” It seemed like science fiction to many, with promises to revolutionize our devices. After all, electrons move much faster in graphene than in silicon, and most of our electronics are silicon-based, so it must be better! Where did all the hype go?
It turns out there’s one big problem with implementing graphene into electronics: it doesn’t have a bandgap – that is, it takes no extra energy to excite the electrons in graphene. Why is that bad? If there is no energetic cost to switching the electrons “on,” then they are effectively always on and can’t be turned off. In contrast, silicon’s bandgap allows for those on and off states, and therefore the 1’s and 0’s that we use in modern computing. Modifying graphene to have a bandgap is tough, so it may not be giving you a cell phone that wraps around your wrist any time soon (and let’s be real: that’s all most folks really care about.) But what can it do?
Since graphene is a 2D material, its surface area is huge, meaning it’s extremely chemically reactive. Because of this, graphene has promise as a water filtration membrane. It’s flexible, incredibly strong, and it also absorbs light well enough that a single graphene layer can be identified with the naked eye. These and many other chemical and optical properties still make graphene relevant in research and technology, even if we hear about it less in the news.

So it’s still around! And 15 years on, researchers are actually making good pace on developing commercial graphene-based technologies. And though it was indeed groundbreaking, Geim’s work on graphene still maintained the element of levity present in his levitating amphibians. The method his group used to make their graphene is called mechanical exfoliation. Sound fancy? It isn’t. Grab a hunk of graphite (pencil lead), put some tape on it, and peel off. There, you’ve got what is most likely a few hundred layers of graphene sitting there for your viewing pleasure.
Peer edited by Manuel Galvan
If it conducts so well, why hasnt it replaced copper and gold in everything from circuit boards to transmission lines?
In terms of computing, the ability for an on/off state is crucially important to how your computer Communicates in a multitude of areas, so until they can really nail down how to modify it to do that, it’s not an ideal replacement.