With the current frenzy around COVID-19, you may be wondering how long it will take before a treatment or cure for this novel disease will be available to the general population. Surely, considering the rising number of confirmed cases and deaths in the United States, scientists must be working around the clock to find a cure. And with so many hands on deck, it can’t be too long before we can start delivering therapies to patients – months, maybe a year at most?
The urgency of COVID-19 will certainly grant all promising treatments accelerated review through drug regulatory agencies, drastically shortening the time it takes from the initial discovery of a drug to its clinical usage. Ultimately however, regulatory agencies like the Food and Drug Administration (FDA) for the US, and European Medicines Agency (EMA) for the EU, exist to ensure that the drugs we discover as scientists are safe for humans and both effective against and selective for a specific disease. These are large responsibilities to shoulder, and because of this, a drug candidate has a lot of hoops to jump through before it can become an approved drug that your local physician can prescribe to you.
Dozens of companies are currently working to bring their COVID-19 vaccine candidates through the clinical pipeline. Despite these efforts, it will likely take several years before a COVID-19 vaccine is FDA approved (the Ebola vaccine, for example, was approved in 2019 when first cases were reported in 2014). This is not an uncommon timeline – COVID-19-specific treatments aside, it often takes around a decade for a drug to be discovered on the benchtop, taken through animal studies, through clinical trials, and given the FDA stamp of approval. And even still, many, many candidates fail at one point or another during this process. We’ve seemingly set up many obstacles for ourselves in the battle against diseases, when of course, they have no regard for our drug development timeline. So you may be wondering why we’ve set up the hoops that we have – rigorous and expensive hoops at that – what those hoops are, and if any of them could be changed to streamline and shorten the time from benchtop to bedside.
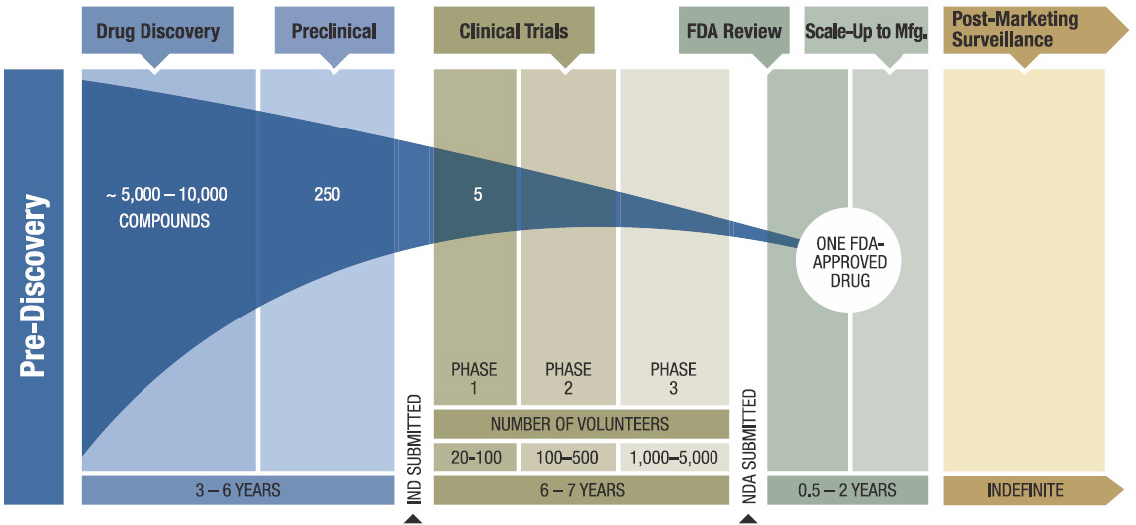
Step 1: Discovery. The first few years of the drug development timeline happen at the benchtop. Generally speaking, molecular biologists, medicinal chemists, and all basic scientists in between work together to identify key proteins implicated in a disease’s mechanism of action and find and optimize molecular compounds that can inhibit those protein(s) in cells. At this early stage, tens of thousands of candidates are made for early testing, but only a handful move forward.
Step 2: Development. Once a subset of promising compounds or “leads” are established, they undergo pre-clinical testing, which takes about 2 years. During this stage, scientists assess the effectiveness of these leads in diseased animal models, determine their optimal doses and best way to administer them, identify potential side effects, and understand how they are absorbed, distributed, and metabolized throughout the body. Based on these findings, researchers can then decide whether to test their candidates in people.
Step 3: Clinical Research. If a compound is effective in treating an animal model of a disease and seems to have minimal side effects, testing can then move into people. In the US, this means that an Investigational New Drug (IND) application is filed with the FDA and, if approved, clinical trials are designed. Clinical trials happen in four phases. In Phase I, researchers give the drug to healthy or diseased volunteers to gain insight into optimal dosing and to understand how the drug is tolerated in the human body. In Phase II, the drug is given to hundreds of diseased patients to assess if the drug is effective in combating the chosen disease. Phase III is effectively Phase II on steroids – the patient population is expanded to thousands and trials are conducted over multiple years to gather data on population efficacy and rare or long-term side effects.
Step 4: FDA Review and Safety Monitoring. If a drug makes it through Phase III trials, drug sponsors typically file a New Drug Application (NDA)* with the FDA, a formal proposition that the FDA approve their drug for sale and marketing. Based on the data in the NDA, the FDA decides whether to approve the drug. The final phase of clinical trials, Phase IV, consists of monitoring and surveillance studies that occur after drug approval.
While the demanding nature of the drug development process is necessary to ensure that the drugs we take are truly helpful, it extends the time needed to bring drugs to market. Not to mention, conducting a drug development campaign is expensive; billions of dollars are invested in any given development program, so it can be a pretty severe blow to drug sponsors if their candidate fails in clinical trials. Some drugs can receive priority designations that can accelerate their approval, but even with this prioritization, the current setup is so expensive and time intensive that it often deters pharmaceutical companies from starting campaigns for high-risk or challenging targets.
Fortunately however, there are emerging technologies in drug discovery that may cut down both the cost and time needed for finding promising drug candidates. Over the years, pharmaceutical companies have built libraries that contain up to hundreds of thousands of compounds, and with investment in automation, they can test thousands of them for efficacy against a protein target via high throughput screening. Some libraries even have genetic tags, where each library member can be identified via an appended barcode. These barcodes allow entire libraries to be tested simultaneously, since promising drug candidates can be singled out and identified with their unique barcode.
On the clinical side, the use of technology is a key part of streamlining trials and making them more efficient. Clinical trial conduct is still often manual and redundant; using modern technology to perform real-time document collection will greatly expedite aspects of study start-up (like contract and budget negotiations, patient recruitment, managing regulatory documents and patient files, etc.) and facilitate data reporting between multiple sites during a trial. There has also been a recent push for adopting adaptive clinical trials, where variables like dosing, study size, and even administered drug(s), can change throughout the course of a trial in hopes of zeroing in on a specific patient population in which a drug is most effective.

Overall, it may be easier to think of it this way – the drug development pipeline is kind of like a gauntlet. New technologies in drug discovery can equip our runners with armor that allow them to better withstand the gauntlet’s attacks, and streamlined clinical trials can help them run faster. With these power ups, we hope that the best runners make it through the gauntlet more quickly, so patients in need can receive novel therapies.
Importantly however, the gauntlet itself remains the same. Rigorous clinical trial testing is needed to ensure that we are in fact creating safe and effective medicines, and this stringency should not be compromised. In the face of public health emergencies, like COVID-19, it may seem like the drug development and approval process is sluggish. But because of its rigor, if the FDA were to approve a treatment for COVID-19 tomorrow, we would trust in the efficacy and safety of that treatment – and that assurance, at least personally, is worth the longer wait time.
*NDAs are only applicable for molecular compounds, like aspirin or ibuprofen. Biologics (i.e. vaccines, insulin, and therapeutic antibodies) go through a somewhat different approval process that isn’t discussed here.
Peer edited by Kayla Goforth & Manuel Galvan