Some of us may have characteristics we would like to change. We may want to be taller, skinnier, or smarter. While many traits can be changed during our lifetime, others simply cannot. There are certain ‘fates’ we cannot avoid, such as inherited diseases. For example, if you inherit two copies of a gene called CFTR that has mutations, you are likely to develop Cystic Fibrosis. Although your life choices can help accommodate the disease, there is currently no cure for Cystic Fibrosis and many other genetic disorders. However, with the development of genome editing, the potential to alter the entirety of our genetic information, there is hope in the ability to one day eradicate genetic diseases once and for all.
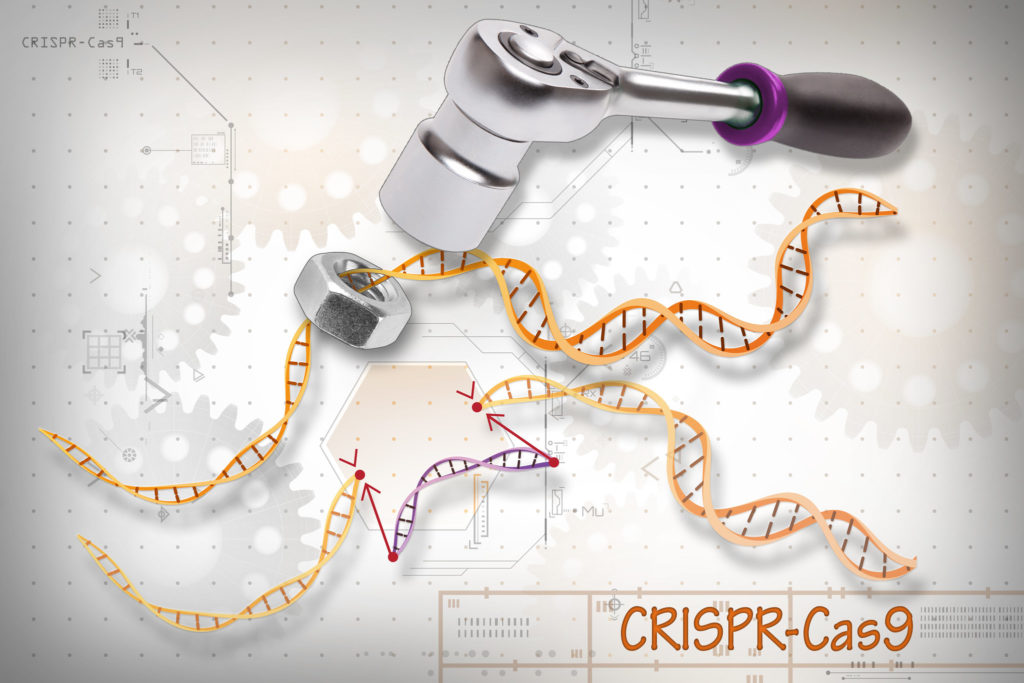
There are currently several genome editing methods available, but the most popular method is the CRISPR-Cas9 system, which was recently and famously used to edit the germline of babies born in China. Editing the germline means that the changes made to the genome will be inherited throughout following generations. Although this practice is still officially banned around the world, CRISPR-Cas9 was used to change two twins’ genomes. This tool allows us to edit parts of the genome by either removing, adding, or changing a given segment of DNA sequence. It is the go-to editing technique used by scientists because it can generate changes quickly, efficiently, and accurately at a relatively low cost. There are two major components of this system – Cas9 and a guide RNA (gRNA). Cas9 is an enzyme that acts as ‘molecular scissors’ and cuts both strands of DNA at a specific location, which allows the removal, addition, or change of DNA sequence. The sequence where Cas9 cuts is determined by the gRNA, which, as its name implies, is an RNA molecule that ‘guides’ Cas9 to a specific place in the genome through complementary base pairing.
The CRISPR-Cas9 system was originally discovered in bacteria as a defense mechanism against viral infection. The most commonly used versions of Cas9 for genome editing come from two bacterial species – Staphylococcus aureus and Streptococcus pyogenes. Both species of bacteria frequently live on the body as harmless colonizers, but in some instances, they can cause disease. If you have been infected with either S. aureus or S. pyogenes, you likely developed adaptive immunity to the bacterium in order to protect yourself against future infections. A recent study from Stanford University published in the journal Nature Medicine examined this phenomenon, asking whether healthy humans possessed adaptive immunity against Cas9 from S. aureus and S. pyogenes.
First off, you may be wondering why adaptive immunity to Cas9 would matter at all. When we are exposed to ‘invaders,’ such as bacteria, our body develops adaptive immunity in the form of antibodies and T cells that target specific components (also called antigens) of the invader. For example, if you’ve eaten raw eggs (in cookie dough, let’s say), you may have suffered from an upset stomach caused by infection with Salmonella. If you are then exposed to Salmonella again in your lifetime, both antibodies and T cells that developed in response to the first infection should act quickly to suppress and clear the infection. Similarly, if you were previously infected with S. aureus, your body may negatively respond to receiving modified human cells producing Cas9 originating from S. aureus for the purpose of genome editing but the antibodies and T cells generated during the initial infection may quickly clear these cells. Our own body may think it is protecting us, although in reality it may be thwarting our efforts to make a change in our genome that is intended to have a positive effect. Even worse, this unintentional stimulation of our immune system could lead to an overwhelming response that can lead to organ failure and potentially death. Unfortunately, there is a documented case of a patient death due to an immune response to gene therapy using viruses. It may therefore be necessary to screen each patient before the start of therapy to determine whether they possess immune components that would make this form of therapy inefficient or even deadly.
The Stanford study mentioned above examined this phenomenon by testing for the presence of both antibodies and T cells against Cas9 from S. aureus and S. pyogenes in healthy donors. The authors found that ~75% of donors produced antibodies against Cas9 from S. aureus and ~60% of donors produced antibodies against Cas9 from S. pyogenes. These results come from analyzing blood from more than 125 adult donors. The scientists also used several approaches to examine T cell responses. In this case, 78% and 67% of donors were positive for antigen-specific T cells again Cas9 from S. aureus or S. pyogenes, respectively.
In summary, the authors of this study provide evidence for existing adaptive immunity to Cas9 from S. aureus and S. pyogenes in a large number of healthy adults. Two additional studies examined adaptive immunity against Cas9 with varying results. However, all three studies suggest that pre-existing immunity to Cas9 needs to be carefully considered and addressed before the CRISPR-Cas9 system moves toward clinical trials as a therapy for genetic diseases. In other words, scientists need to address whether the presence of antibodies and/or T cells against Cas9 would lead to destruction of cells carrying Cas9 for the purposes of genome editing. Other potential solutions include using Cas9 derived from bacterial species that do not cause human infections and thus should not trigger an adaptive immune response, or suppressing the immune system while administering CRISPR-Cas9.
CRISPR-Cas9 genome editing has the potential to transform our lives in many ways. While this technology certainly holds promise for the treatment of genetic disease, the Stanford study highlights the need for more research before we can conclude that CRISPR-Cas9 is effective and safe. Optimization of this system may improve its performance in clinical trials and lead to future approval for the treatment of human disease.
Peer edited by Keean Braceros and Isabel Newsome
This is the first clear description of CRISPR-Cas9 that I’ve ever read! And the message is fascinating and shocking! Thanks