Just two centuries ago, an estimated 70% of people died before the age of 25. In the mid-19th century United States, over 20% of children didn’t make it through their first year! While many public health problems were to blame for this unsettling mortality rate, bacterial infections including tuberculosis, bacterial pneumonia, diphtheria, typhoid, dysentery, and others were disproportionately responsible for high numbers of deaths. The time before World War I is typically referred to as the “medical dark ages,” where a small cut or poorly cooked meal could result in an early grave. Despite the rise of antibiotics and decades of technological breakthroughs, we are now on the precipice of yet another dark age of bacterial infection, this time brought on by the very medicine that saves us. With antibiotic resistant bacterial strains incessantly evolving, how will we find novel therapeutics? And can we do it before it’s too late?
Antibiotic discovery: an abbreviated history
“My only merit is that I did not neglect the observation and that I pursued the subject as a bacteriologist.”
Sir Alexander Fleming, Nobel Prize Speech
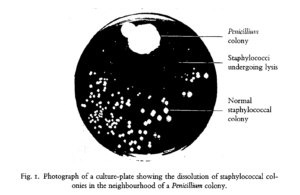
The discovery of antibiotics was arguably the most significant medical achievement of the 20th century. While the late 19th century saw some advances in antibacterial research through the use of fungal and bacterial extracts, these preparations were often inconsistent and highly toxic, limiting their broad use for medical applications. Fast forward to 1928, when Alexander Fleming, a physician by training, returned from vacation to find a mold contamination in one of his petri dishes – a common woe of all microbiology Ph.D. students. However, rather than toss the plate and start over, he observed that the mold had a clear area of agar surrounding it, seemingly untouched by the Staphylococcus (abscess-causing bacteria) he had streaked on the plate prior to his holiday.
Fleming hypothesized that the mold was secreting some form of chemical to prevent bacterial growth. To test this, he isolated and grew the mold intentionally, blending it together to make a “mold juice” that could be applied to other bacterial cultures. The results of these studies were astounding, with the mold juice inhibiting the growth of bacteria that cause staph, strep, diphtheria, and meningitis. While Fleming and his colleagues were unable to successfully isolate the chemical responsible for this effect, chemists at Oxford University successfully isolated penicillin in time to save an estimated 80,000 – 200,000 soldiers in World War II and countless others in the years that followed.
When bugs fight back: the rise of antibiotic resistance
“The time may come when penicillin can be bought by anyone in the shops. Then there is the danger that the ignorant man may easily underdose himself and by exposing his microbes to non-lethal quantities of the drug make them resistant.”
Sir Alexander Fleming, Nobel Prize Speech
Almost immediately after its discovery, Fleming noted that penicillin’s bacterial inhibition was dose-dependent, with smaller doses proving ineffective against some

of the bacteria which could then propagate and overcome the antibiotic in its entirety. While the next 20 years resulted in a “golden age” of antibiotic discovery, the sword of Damocles was already hovering overhead, though its threat was minimized in the minds of scientists. Even in one of the first publications on acquired penicillin resistance, the authors stated, “Syphilis has now been treated with arsenicals for about 40 years without any indications of an increased incidence of arsenic-resistant infections, and this work gives grounds for hoping that the widespread use of penicillin will equally not result in an increasing incidence of infections resistant to penicillin.”
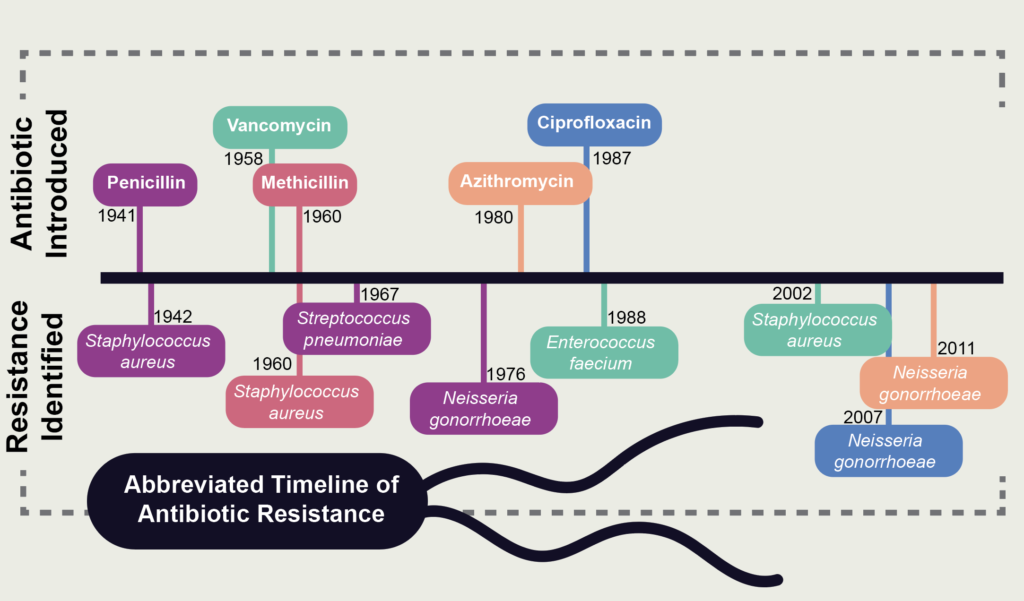
However, their hope was naïve at best. For nearly every antibiotic produced during the so-called “golden age,” resistant bacterial strains were discovered, many within a year of therapeutic usage. A year after penicillin was approved for medical use, penicillin-resistant staph was discovered, joined 25 years later by the discovery of penicillin-resistant strep. When methicillin was released in 1960, it seemed to be a promising alternative to penicillin, yet methicillin-resistant staph (commonly referred to as MRSA) was discovered the same year. Similarly, while wide-spectrum cefotaxime was released in 1980, it took only three years to find cefotaxime-resistant Escheria coli.
As Fleming wisely predicted, the rampant overuse of antibiotics has played a damning role in the prevalence and proliferation of antibiotic resistance. Even in 2010, when the dangers of antibiotic-resistant pathogens had already been firmly established, doctors in the United States were prescribing 500+ antibiotic prescriptions per 1,000 people, with doctors in states such as West Virginia, Indiana, Louisiana, and Alabama prescribing up to 1,200 prescriptions per 1,000 people. Over-prescription of antibiotics led to lack of clinical testing to identify the exact pathogen and its susceptibility to antimicrobials, both of which are exasperated by patient expectations of near-instantaneous diagnosis. Additionally, antibiotics are available for global purchase via the internet, enabling inexpensive access to antibiotics to consumers — even in countries with more rigorous restrictions. Further still, the consistent use of antibiotics in agricultural applications, while important to help increase crop production and decrease food insecurity, has generated profound effects on microbial communities and contributed to the rise of antibiotic resistance in humans.
Today, more than 2.8 million infections, resulting in over 35,000 deaths, are caused by antibiotic-resistant bacteria every year. Even worse, many of the bacteria so successfully fought off in the mid-20th century have evolved into so-called “superbugs,” multidrug-resistant pathogens with higher mortality rates than their less evolved predecessors. In fact, the Infectious Disease Society of America recognized these pathogens as “…one of the greatest threats to human health worldwide.” This is enhanced by the prevalence of hospital-acquired infections. Hospitals, with their continued and required use of antibacterials to maintain sanitary environments, have become a breeding ground for highly dangerous superbugs, which now make up over 15% of hospital-acquired infections in the world. The majority of these infections are caused by “ESKAPE” pathogens, consisting of Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp., which are associated with the highest risk of mortality and highest health care costs. The World Health Organization includes these difficult to treat infections on its list of bacteria that desperately require novel therapies.
Natural products for antibiotics: our only hope?
“Nature makes penicillin… I just found it.”
Sir Alexander Fleming
Since the time of ancient civilizations, humans have used plant-based medicine for the treatment of a variety of ailments, including that of bacterial infection. In fact, researchers from the University of Nottingham and Texas Tech recently identified a medieval remedy for staph in a 10th century Anglo-Saxon leechbook. When they recreated the remedy, it successfully treated both staph and the more-deadly MRSA. If the Anglo-Saxons could invent a recipe with antibiotic capabilities, what else can we learn from our ancient predecessors?
Enter antimicrobial peptides, naturally formed peptides (small proteins) found in all forms of life. These peptides are diverse in both size and sequence, but have conserved chemistries that allow either binding or traversing of bacterial membranes. Interestingly, many of the identified antimicrobial peptides are broad spectrum, meaning they work against a variety of bacteria, as their non-specific mechanisms of action prevent selective evolution of resistance from target pathogens. To date, natural products have been the single most successful source of active compounds for drug discovery.
Whether or not we will be able to produce enough novel antibacterial therapeutics to prevent the oncoming “dark age” of bacterial infection related fatalities remains to be seen. However, with researchers all over the world consistently seeking out new therapies, there is room to be optimistic. At UNC, researchers in the Hicks Group have developed a pipeline to rapidly identify novel antimicrobials, enabling the discovery of bioactive peptides in violets, spinach leaves, and bacterial and fungal secretions. The Bowers Group takes a different approach: rather than discovering novel peptides, they use genetic manipulation to alter naturally occurring pathways to engineer microbial factories that can synthesize useful antibiotics, thus generating their own repertoire of bioactive small molecules. Additionally, the UNC Eshelman School of Pharmacy’s Natural Products Research Laboratories, led by Dr. Kuo-Hsiung Lee, has designed and discovered several thousand bioactive pharmaceuticals since its founding in 1971, including anti-viral and anti-cancer agents. Through continued dedication to interdisciplinary scientific approaches and an enhanced commitment to responsible antibiotic use, we can usher in a new golden age of antibiotics that sustains us through the centuries to come.
Peer edited by Elise Hickman and Colleen Lawrimore
Very interesting article. Enjoyed reading it. Enlightening also.