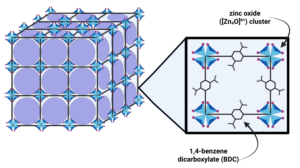
Picture a crystal lattice, razor-sharp and molecularly exact, so vivid that you can visualize the atoms themselves with an electron microscope. The crystalline material is so precise that a single speck can have a surface area thousands of times larger than its own volume. First discovered in 1989 by Richard Robson, metal organic frameworks (MOFs) are a class of porous polymers that act as molecular sponges. They can harvest water from dry air, trap pollutants with surgical precision, and exchange gases through their cavernous, tunable pores. Susumu Kitagawa from Kyoto University, Richard Robson from the University of Melbourne, and Omar Yaghi from the University of California Berkley received the 2025 Nobel Prize in Chemistry for developing this new type of molecular architecture.
Kitagawa’s motto, that even useless things can become useful, guided a unique scientific pursuit. His patient approach of slowly building seemingly impractical structures led to transformative technologies. The journey of MOFs started with Richard Robson, who realized that the negative space positioned between atoms and the alignment of chemical bonds held an incredible amount of information. Robson questioned whether it might be possible to leverage the negative space by linking specific atoms and molecules together to form new structures.
In 1989, in the Journal of American Chemical Society 1989, Robson presented his novel chemical reaction where he combined copper ions with the molecule 4′,4″,4”’,4””-tetracyanotetraphenylmethane, essentially a four-arm structure with terminal nitrile groups. This reaction produced a crystalline structure with massive cavities, marking the start of a new way to construct materials.
Robson’s initial frameworks, while innovative, were unstable. Through constant refinement, Kitagawa developed a 3D structure using cobalt, nickel, or zinc paired with a molecule called 4,4′-bipyridine. This new 3D MOF consisted of interconnected channels that could be dried of water and later refilled with gases such as methane, nitrogen, and oxygen without changing shape. The combination of mechanical stability and reversible porosity proved a dream. It revealed that when the crystal structures align precisely, they can behave like molecular sponges, hosting and releasing compounds as needed. What began as a laboratory curiosity evolved into a platform for countless future applications.
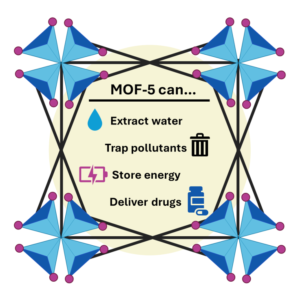
The next major advance came in 1999 when Omar Yaghi showcased MOF-5 to the world. Built from zinc oxide clusters linked by terephthalic acid, an organic compound which serves as the linker between the zinc oxide clusters, MOF-5 exhibited an astonishing surface area to volume ratio of 2,200 m2/cm3. MOF-5 was both flexible and shapeable and presented the world with applications ranging from removing PFAS from water to filtering air pollution and capturing greenhouse gases such as methane and carbon dioxide. With MOF-5 as the starting point, MOFs are now becoming more customizable, where metal MOF nodes and organic linkers can be swapped to create materials with specific, tunable properties.
The 2025 Nobel Prize in Chemistry went to three scientists who spent years, unbeknownst to the world, quietly optimizing and assembling atomic-scale architectures. These molecular materials influence systems that extend far beyond their microscopic size, impacting global challenges in energy, water, and pollution. Their success demonstrates how atomic-scale designs can solve global-scale problems, pointing towards bolder solutions, one crystalline structure at a time.
Illustrated by Claire Miller
Peer Edited by Lauren Griffith and Brittanie Winfield